In order to effectively protect consumers, markets and manufacturers from counterfeits, a legally binding framework for tracing prescription-only medicines was created, for example, with the EU Falsified Medicines Directive (FMD) of 2011 and the Delegated Regulation (EU) 2016/161. Among other things, data matrix codes, database comparisons with national verification systems and solutions for detecting the integrity of product packaging are used. A central element in the fight against counterfeit medicines is digitisation. The Arvato CSDB is used as a proven serialisation solution by over 80 pharmaceutical companies and ensures both a smooth serialisation process and the seamless implementation of legal requirements. We are also an official Blueprint Service Provider of the European Medicines Verification Organisation (EMVO). More Information: https://arvato-systems.com/arvatocsdb

Product Keywords
IT Solutions-Software
More Solutions:
Vaccination Management for Public Health Authorities and Companies
Arvato Systems GmbH
Communication in medicine with kim +
Arvato Systems GmbH
Arvato Systems GmbH
Arvato Systems GmbH
Related Solutions:
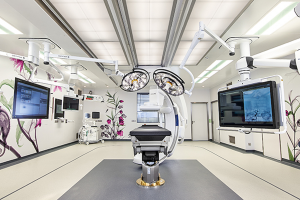
Hospital Equipment
Getinge Deutschland GmbH
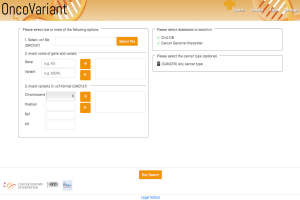
OncoVariant
BioVariance GmbH
Automatic X-ray film processors
PROTEC GmbH & Co. KG
Software Option “Clinical Assistance Package”
Draegerwerk AG & Co. KGaA