Innovative reagents and new instrument technology from Cytecs enable considerable cost reductions and the widespread use even in regions with poor infrastructure / Approval as IVD product already obtained
Münster, Germany – July 29, 2020: German diagnostics company Cytecs announces the introduction of a new test for the molecular diagnostic detection of the coronavirus SARS-CoV-2, causing the Covid-19 infectious disease.
The new test has already been successfully registered via Münster district government in accordance with the EU regulation on in vitro diagnostics (IVD) and has thus been officially approved as an IVD product in Germany and most countries worldwide since July 20, 2020. The comparative studies to be submitted in the context of this notification prove the equivalence of the quality and reliability of the results of the test now newly introduced by Cytecs in comparison to the conventional so-called “real-time RT-PCR” methods.
With the new method, a significant reduction of the total reagent costs per test to no more than 8 Euro is achieved, while the costs for the associated instrumentation of the innovative and portable molecular diagnostic technology, also newly developed by Cytecs, amount to only 10,000 Euro. The sample throughput is up to 600 tests per day. Based on the highly affordable instrument costs, a multiplication of the sample throughput is very easy to achieve.
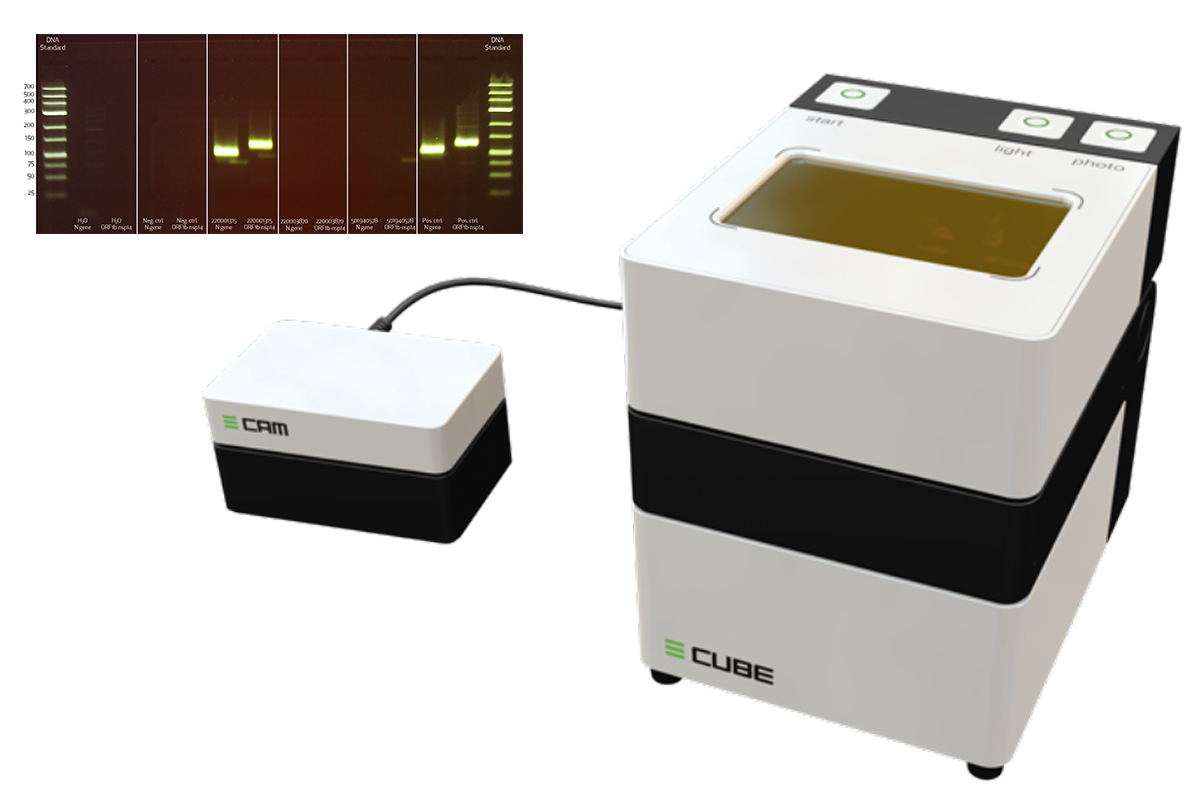
The method is based on the amplification of two SARS-CoV-2 gene sequences after transcription of the viral RNA into the complementary DNA. This DNA is then labelled with a DNA-specific fluorescent dye and analysed using Cytec’s newly developed ultra-compact “all-in-one” gel electrophoresis system E-CUBE which is also suited for a decentralized use. While the same molecular biological approaches are used for the Cytecs test as for conventional tests using the real-time RT-PCR method, the cytochemical detection of the nucleic acid amplificates is new.
In addition, the otherwise extremely temperature-sensitive reagents for SARS-CoV-2 detection are now available for the first time as lyophilized, i.e. freeze-dried substances. Cold chains and cold storage are no longer necessary on the basis of the new Cytecs test, which eliminates the previously common, very problematic challenges when shipping and using conventional molecular diagnostic reagent kits in remote as well as in warm regions of the world.
Another advantage of the new method is that the necessary conventional PCR devices (“thermocycler”) and gel electrophoresis instruments are already part of the standard equipment of most laboratories in research institutions and larger hospitals – in industrialized countries as well as in sub-Saharan Africa, for example. In many cases, there is no need to purchase new analytical instruments for Cytecs’ new SARS CoV-2 test and, when using existing instruments or Cytecs instrument technology, there is no need to send technicians to install equipment.
Currently, the World Health Organization (WHO) reports over a quarter million new corona infections worldwide per day. The extremely dynamic expansion of the pandemic affects poorer countries in particular, in which the health systems were hardly prepared for such a development and, with regard to the urgently required laboratory infrastructure and equipment, cannot be retrofitted in time to meet the needs of the population.
The development of the new test from Cytecs was primarily aimed at addressing the problem that most developing and emerging countries do not yet have sufficient test capacities to combat and control the coronavirus pandemic. In this context, Professor Dr Wolfgang Göhde, Managing Partner of Cytecs GmbH, has 20 years of experience in the development of new diagnostic concepts for major infectious diseases, particularly in countries with weaker infrastructure and poorer countries. Together with biologist Dr Dr Hildegard Göhde, Wolfgang Göhde founded the company Partec GmbH in Münster in 1967. In 2000, together with his son Roland Göhde, he founded the diagnostics company, which became Partec GmbH in Görlitz in 2011. Since 2000, Partec has been able to offer the lifelong immune status diagnostics that are indispensable for the successful treatment of HIV/AIDS-infected people. This was achieved through new, innovative and, for the first time, mobile device technologies and novel reagents – reduced by a factor of 20 compared to the tests commonly used at that time – at such a low price that this diagnostics became accessible and affordable for all those affected. As a result, Partec was able to equip well over 100 countries, particularly in Africa and Asia, with the corresponding laboratory technology and to supply up to 5 million cost-effective tests per year. The intensive on-site experience gained over many years was the basis and motivation for now developing and establishing an innovative diagnostic solution that enables highly sensitive, reliable detection of the coronavirus SARS-CoV-2 even under difficult laboratory conditions.
Contact:
Cytecs GmbH
Im Derdel 8
DE-48161 Münster | Germany
Tel: +49 (0) 2534 97736-0
Fax: +49 (0) 2534 97736-29
info@cytecs.com