The medical device market is one of the most regulated industries today. More and more country-specific regulations and laws are making it increasingly difficult for manufacturers to expand abroad.
With its unique online database Daedalus®, RegIntA GmbH from Altenstadt in the Rhine-Main area supports entrepreneurs in complying with international regulations, relieves the Regulatory Affairs departments and thus creates legal certainty for medical device manufacturers.
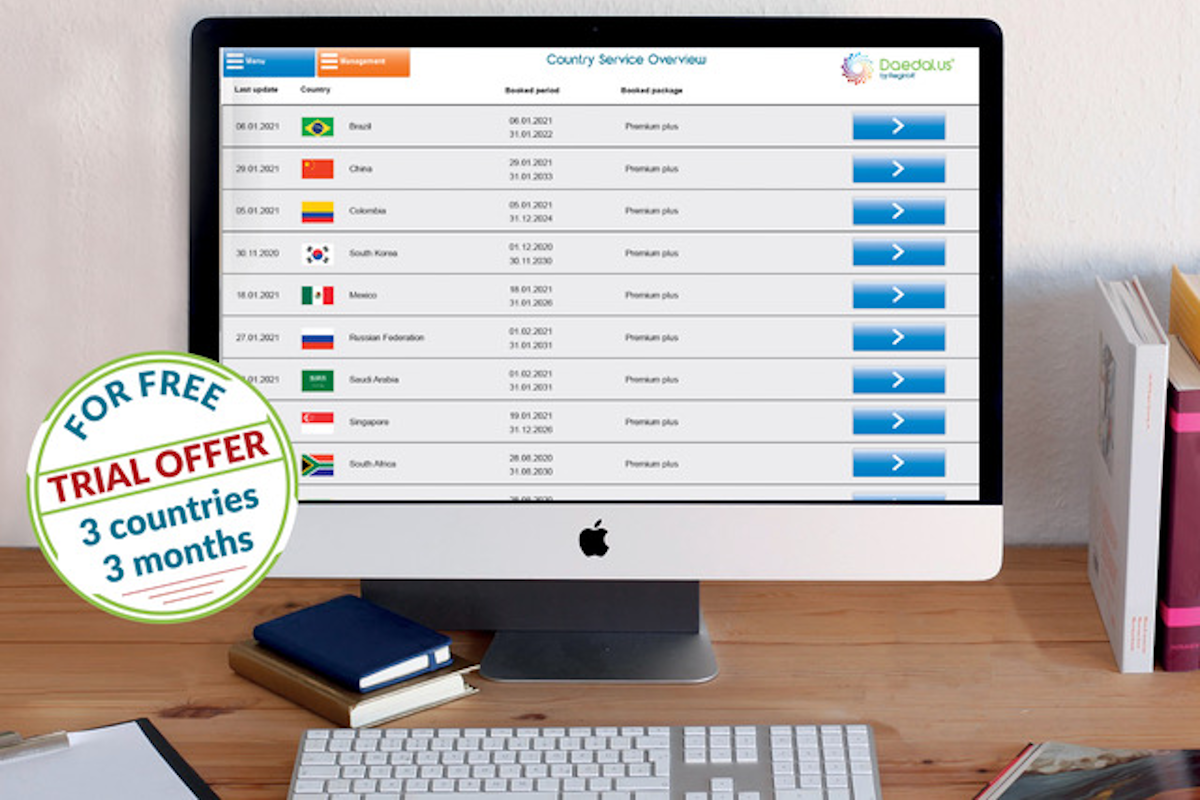
Daedalus® provides manufacturers with an easy-to-understand, up-to-date and country-specific overview that helps companies meet the regulatory requirements in the respective markets. Regulatory Affairs departments, which have access to the comprehensive know-how of international regulations, thus save time-consuming and cost-intensive research of their own. Errors in the critical approval phase are avoided, so that products reach the market more quickly.
Daedalus® also supports companies with change notifications and renewal applications covering the entire product cycle.
RegIntA continuously updates booked information, which clients can access 24/7 via an online portal. Maximum flexibility is offered through packages with different performance features. The countries can also be freely selected.
Currently, RegIntA is presenting a trial offer that gives all interested manufacturers the opportunity to test Daedalus® for three countries for three months free of charge: https://www.reginta.de/registration.html